
Resonance is part of valence bond theory.
Resonance structures are different Lewis structures for a species, written without violating the conventional rules of bonding.
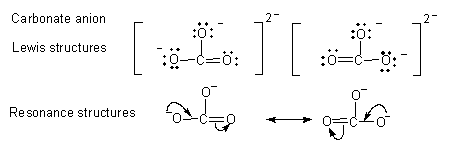
Note: Only two structures have been shown in each case.
- In Lewis structure the valence electrons of all the atoms have to be shown. Charge indicates loss or gain of electrons.
- In resonance structures all valence electrons need not be shown.
- Resonance structures differ in distribution of electron density; the atoms retain their relative positions.
- Movement of electrons is shown using curved arrows with appropriate change in charge.
- The arrows show the direction of electron movement.
- The arrow starts from an electron rich zone and move towards an electron deficient zone never the other way.

- The starting and ending of the arrow is very important, the shape is not and is left to the artistic skills of the writer.
- If the arrow starts at the middle of the bond it implies two electrons constituting the bond are shifting.
- If the arrow ends between two atoms it implies a covalent bond is formed between them.
- If the arrow starts between atoms and ends on one, it means both electrons will reside on that atom.
- This may change the charge. One electron shift is indicated by a fish hook arrow (in free radical reactions).
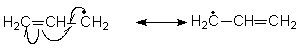
If a structure has a negative charge start shifting electrons from there.
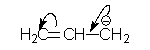
If it has a positive charge move towards it.
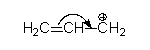
If it is neutral and has an atom with a lone pair start from there.
- The electron shift from one p-orbital to another should not violate the basic rules, like no orbital can have more than two electrons.
- The contribution of each individual resonance structure towards the final hybrid is not necessarily same.
- The structures which have charge separation are less stable compared to the neutral forms hence their contribution is less towards the hybrid.
- For acetate anion the negative charge is always on oxygen, also the two structures are symmetrical. So both contribute equally to the final hybrid.
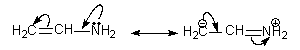
- In the following structures of aminopropene the second structure has charge separation and is less stable hence contributes less while the first is neutral more stable and contributes more towards the hybrid.
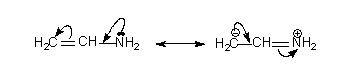
To get different resonance structures either a one arrow (two electrons) or two arrow shifts can be used again without violating the rules.
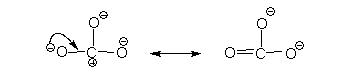
In the above resonance structure a single arrow shift has been used. This results in a structure without violating any rules.
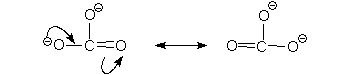
In the above scheme if only a single arrow shift is used then carbon will have five bonds which is not appropriate, a two arrow shift is a must.